Which Statement Best Describes The Change in Entropy During The Synthesis of A Polymer? The change in entropy during the synthesis of a polymer is considered to be 0.5, when calculating the entropy change of a polymer molecule.
The increase in entropy is because the monomer becomes more ordered at low monomer concentrations and less ordered at high concentrations.
This means that the polymerization process could not become random or have no structure because it must retain its specific structure from one end to another.
The change in entropy is the largest that can occur due to production of new minmum free energy.
In this case, the free energy is lower than the inclusion enthalpy, therefore the polymerization results in a negative change in entropy because it must get more disordered.
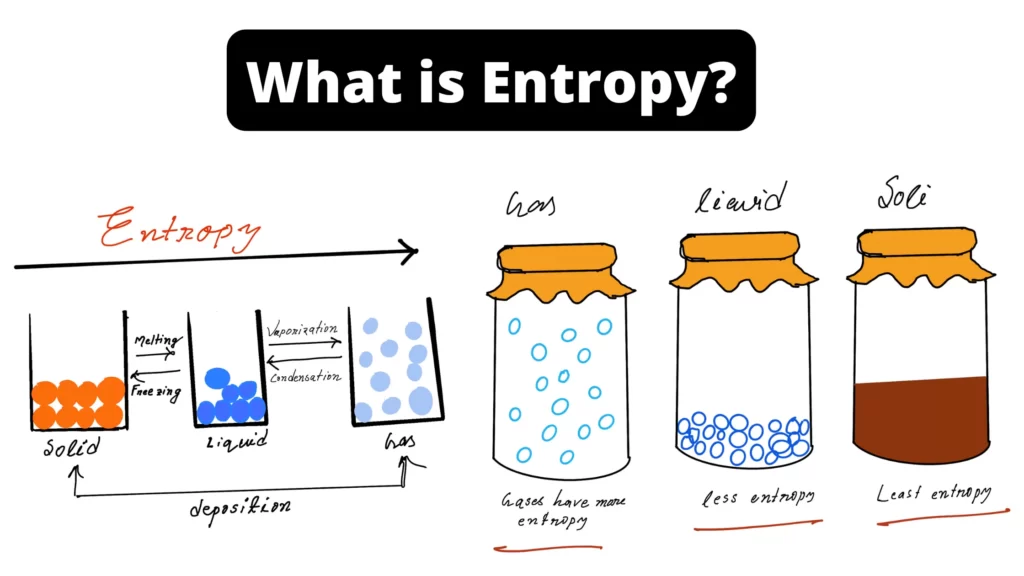
How to describe entropy?
Entropy is a measure of the number of ways that you can reorganize the monomer to make a polymer.
In most cases, during the synthesis of a polymer: the entropy decreases.
It is usually positive, but can be negative if an increase in entropy occurs because less randomness exists for the system.
If it’s an increase in entropy, then the monomer is more ordered (less random) than before the polymerization occurred.
The change in entropy is relative, and depends on whether the polymerization is endothermic or exothermic.
If the polymerization is going to be exothermic, then it means that the starting materials will have more energy than they take as heat while they are reacting.
The idea of entropy provides a mathematical manner to encode the intuitive notion of which processes are not possible, even though they could no longer violate the fundamental law of conservation of strength.
As an instance, a block of ice located on a warm range absolutely melts, even as the range grows cooler.
Such a technique is called irreversible because no slight exchange will reason the melted water to show back into ice even as the stove grows warmer.
In contrast, a block of ice placed in an ice-water bathtub will either thaw a little more or freeze a touch extra, relying on whether a small quantity of heat is introduced to or subtracted from the gadget.
Procedures of entropy that last
One of these procedure is reversible because best an infinitesimal amount of heat is wanted to exchange its course from revolutionary freezing to innovative thawing.
In addition, compressed gasoline constrained in a cylinder should both extend freely into the atmosphere if a valve were opened (an irreversible system.
Or it can do useful paintings with the aid of pushing a transportable piston towards the pressure needed to confine the gas.

